Tris(Dibenzylideneacetone)Dipalladium-Chloroform Adduct
| Identification | ||
| Name |
|
Tris(dibenzylideneacetone)dipalladium-chloroform adduct |
| Synonym |
|
N,N,N',N',N'',N''-Hexaallyl-1,3,5-triazine-2,4,6-triamine; Pd2(dba)3.CHCl3; Tris(dibenzylideneacetone)dipalladium(0)-chloroform Adduct; Dipalladium-tris(dibenzylideneacetone)chloroform complex; Tris(dibenylideneacetone)dipalladium-chloroform; (C6H5CH=CHCOCH=CHC6H5)3Pd2 · CHCl3; |
|
|
||
| Molecular Structure |
|
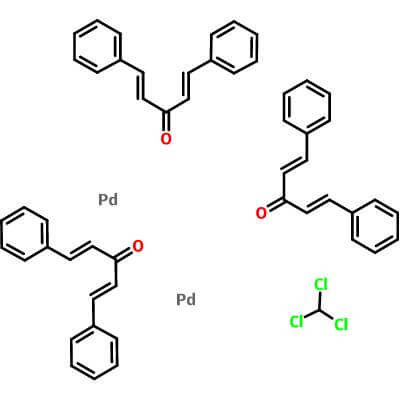 |
|
|
||
| Molecular Formula |
|
C52H43Cl3O3Pd2 |
| Molecular Weight |
|
1035.09 |
| CAS Registry Number |
|
52522-40-4 |
| Pd content |
|
20.4% |
| Properties | ||
| Melting point |
|
131-135 ºC |
| Appearance |
|
Purple Powder |
| Safety Data | ||
| Hazard Symbols |
|
Xi |
| Risk Codes |
|
R36/37/38 |
| Safety Description |
|
S26;S37/39 |
Tris(dibenzylideneacetone)dipalladium-chloroform adduct purposes:
As a catalyst, used in Suzuki, Kumada, Negishi, Buchwald and other coupling reactions in α, β unsaturated ketone precious metal complex such as tri (dibenzylacetone) two palladium - chloroform admixture (i.e., PD2DBA3.CHCl3) in many chemical methods as a catalyst or catalyst system components.
Used as catalyst in coupling reaction of Suzuki, Kumada, Negishi, Buchwald, etc.
Tris(Dibenzylideneacetone) Dipalladium-Chloroform Adduct preparation:
Tris (dibenzylacetone) dipalladium-chloroform adducts are usually prepared from palladium chloride (Ⅱ). CN201180009977.4 provides an optional method for preparing tri (dibenzylacetone) dipalladium (chloroform). This method is convenient, economical, effective and can be implemented on a large scale.
(1) Preparation of Li2PDCl4 solution Palladium
Dichloride (200.2g), lithium chloride (108g) and ethanol (5400ml) were added together in a nitrogen atmosphere, and the solution was stirred overnight at room temperature. Filter the Li2PDCl4 solution and wash the insoluble material with ethanol (50 100ml).
(2) Preparation of tris (dibenzyl acetone) dipalladium-chloroform adducts
Dibenzyl acetone (414.3g, 3.20 molar ratio), ethanol (8360 mL), CHCl3(2250 mL) and sodium acetate (720.4g) were added in a nitrogen atmosphere, and the solution was stirred. The Li2PDCl4 solution is degassed under nitrogen and added to the DBA solution within 30 minutes at the temperature of 50.6 53.2℃. After adding Li2PDCl4 solution, the reaction conditions were maintained for another hour and the temperature was maintained at 50.9 52.3 ° C. Allow the reaction mixture to cool to room temperature while stirring. The reaction mixture was filtered and the product was filtered in deionized water (3000ml) and dried in a vacuum under nitrogen for 30 min. The result is a dark purple solid with some visible white sodium acetate. The solid was combined with deionized water (11000ml). Stir the solution under nitrogen for 15 minutes and strain. The dark purple solid was filtered with deionized water (1500ml) and ethanol (1500ml). The solids were vacuum dried under nitrogen for 30 minutes. The solid was combined with ethanol (1800 mL) and CHCl3(900 mL) and the solution was stirred. The reaction mixture was filtered and rinsed with ethanol (50ml). The solids are vacuum dried under nitrogen for one hour. The product is placed in a glass tray and dried in a vacuum at 25 35 ° C and 15 inches of mercury pressure for 16 hours to a constant weight, resulting in the title product (97.76%; Melting point 125 ‑ 130 ℃).