| Identification | ||
| Name |
|
Sodium hexachloroplatinate(IV) hexahydrate |
| Synonym |
|
Platinum(IV) sodium chloride, Sodium platinic chloride |
|
|
||
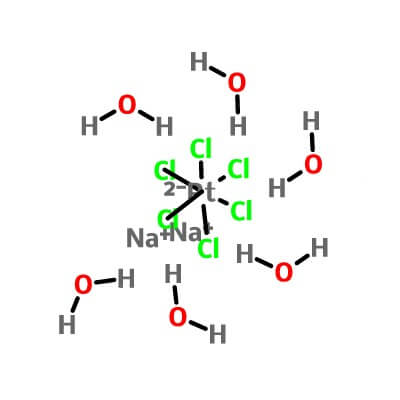
| Molecular Structure |
|
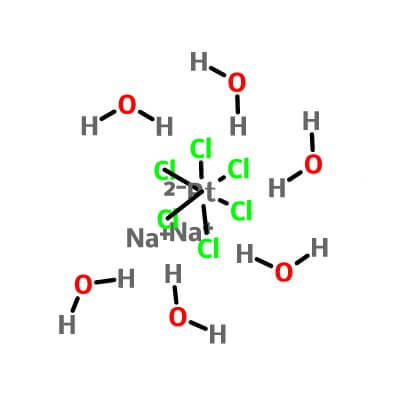 |
|
|
||
| Molecular Formula |
|
Na2PtCl6.6(H2O) |
| Molecular Weight |
|
561.86 |
| CAS Registry Number |
|
19583-77-8 |
|
|
|
|
| Properties | ||
|
Melting point |
|
110℃ -6H{2}O |
|
Density |
|
2.50 |
|
form |
|
Crystalline Solid |
|
colour |
|
Orange |
|
proportion |
|
2.5 |
|
stability |
|
hygroscopic |
| Water solubility |
|
soluble |
| Safety Data | ||
| Hazard Symbols |
|
Xn |
| Risk Codes |
|
R42/43 |
| Safety Description |
|
S22;S24;S37 |
|
HazardClass |
|
6.1 |
|
Product Number |
|
28439000 |
Sodium hexachloroplatinate(IV) hexahydrate application:
Sodium hexachloroplatinum can be prepared by reacting sponge platinum with industrial sodium chloride and passing ammonia gas. It can be used to prepare platinum nitrate solution and platinum nitrate compound. It is a kind of catalyst precursor product that is very popular in the market.
Sodium hexachloroplatinate(IV) hexahydrate preparation:
The medium temperature molten salt of sponge platinum is chlorinated to produce sodium chloroplatinate: Mix 300g of commercially available sponge platinum powder with a purity greater than 99.95% and 900g of industrial sodium chloride at a mass ratio of 1:3, and then load the mixed material into Chemicalbook. The quartz boat is heated to 450℃ in a tube furnace, and then chlorine gas is introduced. The chlorine gas flow rate is 65mL/min. After chlorine gas is introduced for 4 hours, the chlorine gas is disconnected. After cooling to room temperature, take it out to obtain sodium hexachloroplatinum powder.