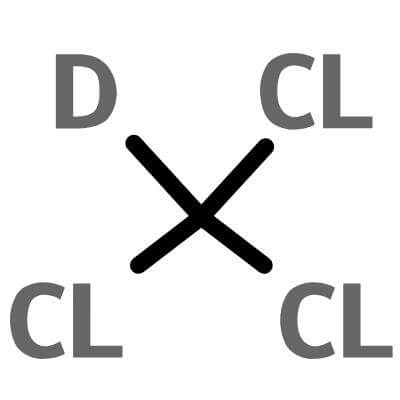
deuterated chloroform , 865-49-6 , CDCl3
| Identification | ||
| Name |
|
deuterated chloroform |
| Synonyms |
|
Methane-d,trichloro Chloroform,deutero deuteriochloroform (2H)Chloroform trichloromethane-d1 Chloroform-d Deuterotrichloromethane Trichloro(2H)methane trichloro-deuterio-methane |

|
||
| Molecular Structure |
|
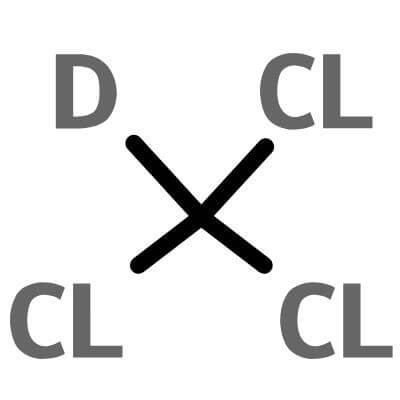 |
|
|
||
| Molecular Formula |
|
CDCl3 |
| Molecular Weight |
|
120.38400 |
| CAS Registry Number |
|
865-49-6 |
|
EINECS |
|
212-742-4 |
| Properties | ||
| Appearance |
|
Colorless liquid |
|
Density |
|
1.5 |
|
Boiling point |
|
60.8ºC |
|
Melting point |
|
-64ºC |
|
Flash point |
|
62ºC |
|
Abundance |
|
99% |
| Safety Data | ||
| Hazard Symbols |
|
Xn |
|
Risk Codes |
|
R22;R38;R40;R48/20/22 |
|
Safety Description |
|
S36/37 |
|
Transport Information |
|
UN 1888 |
|
Customs code |
|
28459000 |
Deuterated Chloroform Usage and Synthetic Method:
Deuterated chloroform (CDCl3) is a deuterated product of chloroform, a deuterated solvent. Colorless liquid. Used for NMR; reagents for nuclear magnetic instruments. The reason for its use is that it has good solubility to the sample; its residual signal peak will not interfere with the signal peak of the sample. The residual proton signal of deuterated chloroform is located at 7.26ppm; the possible residual water peak is at 1.56ppm.
Deuterated chloroform is a reagent for nuclear magnetic equipment; organic synthesis raw material, mainly used to produce freon (F-21, F-22, F-23), dyes and drugs, and is often used as an anesthetic in medicine. It can be used as a solvent and extractant for antibiotics, spices, oils, resins, and rubber. Mixed with carbon tetrachloride can be made into non-freezing fireproof liquid body. It is also used as a propellant for aerosols, a fumigant for grains and a standard liquid for temperature calibration. Industrial products usually add a small amount of ethanol to make the generated phosgene react with ethanol to produce non-toxic diethyl carbonate. Before using industrial products, you can add a small amount of concentrated sulfuric acid, shake, wash with water, and dry with calcium chloride or potassium carbonate to obtain ethanol-free chloroform.
Chemical shift-NMR analysis:
In hydrogen nuclear magnetism, the chemical shift of deuterated chloroform is 7.26 ppm. When using deuterated reagents without standards (tetramethylsilane), the peaks of deuterated chloroform can be used for calibration. In the carbon spectrum, it is a common method to use the middle peak of the triplet of deuterated chloroform as the calibration peak, but there are different records about the shift of this middle peak, such as in J.Org.Chem.1996,61, In 3417-3422, the shift of this peak is set to 77.0ppm .
(ChemicalshiftsarereportedrelativetothecentralpeakofCDCl3at77.0ppm). In J.Org.Chem.1997,62,7512-7515, the author studied the chemical shifts of common solvents in detail. Here, The chemical shift of deuterated chloroform was recorded as 77.16±0.06 ppm. Under normal circumstances, the deviation of the chemical shift of the carbon spectrum is relatively small. It can be considered that the chemical shift (middle peak) of deuterated chloroform is 77.23ppm. This value is not an absolute authoritative value and is only for reference.
Deuterated chloroform water peak:
The position of the water peak in different deuterated reagents is different. The water peak of heavy water is about 4.67 ppm. There is a trend, the more the amount of water, the lower the field. Deuterated chloroform and water are less soluble, and the water content is lower, so Near 1.59ppm. Deuterated acetone is about 2.8ppm, and the water peak of deuterated dimethyl sulfoxide is about 3.4ppm. If water is added to deuterated acetone, the water peak will gradually move to the low field, and finally stop at about 4.7ppm.