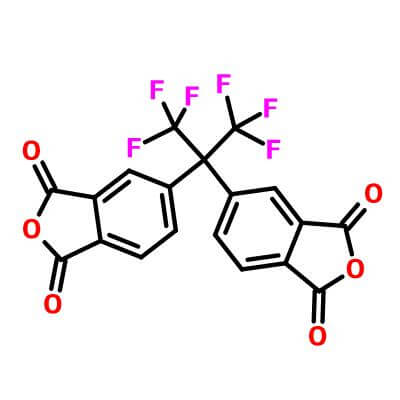
4,4'-(hexafluoroisopropylidene)diphthalic anhydride_ CAS:1107-00-2
Quality 4,4’-(hexafluoroisopropylidene)diphthalic anhydride,1107-00-2,6FDA suppliers & exporter - all products made in China.
4,4'-(hexafluoroisopropylidene)diphthalic anhydride_ CAS:1107-00-2
| Identification | ||
| Name |
|
4,4’-(hexafluoroisopropylidene)diphthalic anhydride |
|
Synonyms |
|
2,2-Bis(3,4-dicarboxyphenyl)-1,1,1,3,3,3-hexafluoropropane 4,4′-(Hexafluoroisopropylidene)bis(phthalic anhydride) 4,4′-(Hexafluoroisopropylidene)diphthalic anhydride 6FDA |
|
|
||
| Molecular Structure |
|
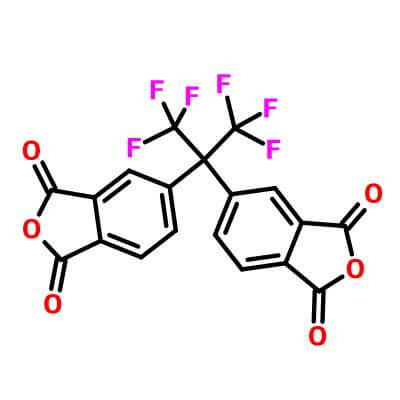 |
| Molecular Formula |
|
C19H6F6O6 |
| Molecular Weight |
|
320.23 |
| CAS Registry Number |
|
1107-00-2 |
|
Electronic level |
||
| Melting point |
|
244-247 ºC |
| Appearence |
|
off-white crystal powder |
| Purity |
|
99.5% min |
|
Boiling Point |
|
494.5 ºC at 760 |
| Flash Point |
|
243.7 ºC |
| Security Information | ||
| Dangerous goods mark |
|
C,Xi |
|
Hazard category code |
|
34 |
|
Dangerous goods transportation number |
|
26-36/37/39-45 |
Introduction to 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride:
Hexafluorodianhydride (that is, 4,4'-(hexafluoroisopropylene) diphthalic anhydride, 6FDA) is one of the six most widely used dianhydride monomers, and it is also currently a colorless transparent polyamide The dianhydride monomer with the largest amount of imines. The glass transition temperature of polyimide synthesized from hexafluorodianhydride is usually above 300°C, and the mechanical properties and electrical properties are well balanced. So far, it is still the most representative fluorine-containing polyimide.
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride use:
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride is an organic synthesis intermediate and pharmaceutical intermediate. It can be used in the laboratory research and development process and the chemical and pharmaceutical synthesis process, mainly as an electronic material polymer.
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride preparation:
There are usually two methods for preparing the corresponding aromatic dianhydride from the intramolecular dehydration of aromatic tetraacids:
(1) Physical method: that is, heating the aromatic tetraacid to melt, and then performing intramolecular dehydration to obtain the aromatic dianhydride;
(2) Chemical method: add aromatic tetraacid to excess acetic anhydride or acetic anhydride/acetic acid mixed solvent, heat to dissolve, and then cool to precipitate aromatic dianhydride.
The dianhydride obtained by the physical method is a molten block with a darker appearance. Generally, needs to be crushed before use; the dianhydride obtained by the chemical method is crystalline with a lighter appearance, but the yield of the dianhydride is lower than that of the physical method. . These two methods can be applied to the other five other than hexafluorodianhydride (ie pyromellitic dianhydride PMDA, biphenyl tetracarboxylic dianhydride BPDA, diphenyl ether tetracarboxylic dianhydride ODPA, benzophenone Tetracarboxylic dianhydride BTDA and bisphenol A dianhydride (BPADA) are the most widely used dianhydride monomers.
Related Product