| Identification | ||
| Name |
|
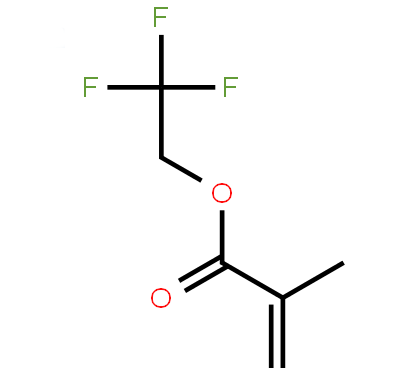
2,2,2-Trifluoroethyl methacrylate |
| Synonyms |
|
1,1-Dihydroperfluoroethyl methacrylate; 2-Methyl-2-propenoic acid 2,2,2-trifluoroethyl ester Trifluoroethyl methacrylate; 2,2,2-tifluoroethyl methacrylate; 2,2,2-Trifluoroethyl 2-methyl-2-propenoate;
Acryester 3FE; |
|
|
||
| Molecular Structure |
|
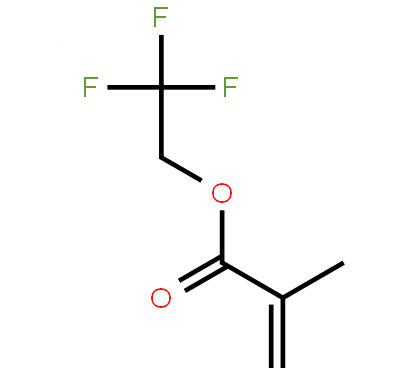
|
|
|
||
| Molecular Formula |
|
C6H7F3O2 |
| Molecular Weight |
|
168.11 |
| CAS Registry Number |
|
352-87-4 |
|
EINECS |
|
206-525-3 |
| Properties | ||
| Density |
|
1.181 |
| Boiling point |
|
59 ºC (100 mmHg) |
| Refractive index |
|
1.36-1.362 |
| Flash point |
|
16 ºC |
| Safety Data | ||
| Hazard Symbols |
|
F;Xi |
| Risk Codes |
|
R11;R36/37/38 |
| Safety Description |
|
S26;S37/39 |
| Transport Information |
|
UN 3272 |
Mainly used in the production of thermosetting resins, hydroxy acrylic resins, adhesives, fiber treatment agents and modifiers for synthetic resin copolymers.
It is mainly used in coatings to improve its weather resistance, water resistance and pollution resistance. It can also be used as the cladding and core material of optical fibers, contact lenses and computer toners.
Mainly used in coatings to improve weather resistance, water resistance and pollution resistance. It can also be used as a charge regulator for optical fiber cladding and core materials, contact lenses and computer toners and carrier particles.
Preparation of 2,2,2-Trifluoroethyl methacrylate:
The invention discloses a preparation method of trifluoroethyl methacrylate. Using trifluoroethanol and methacryloyl chloride as raw materials, the esterification reaction is carried out under the protection of polymerization inhibitor, and then the target product trifluoroethyl methacrylate is collected from the reaction product. The purity of the trifluoroethyl methacrylate obtained by the preparation method of the invention is greater than 99%, the yield is greater than 80%, and the boiling point is 100.8-101.2°C. Compared with the prior art, the present invention has mild reaction process conditions.