| Identification | ||
| Name | 2-Hydroxyethyl methacrylate | |
| Synonyms |
Ethylene glycol methacrylate;
Glycol methacrylate; HEMA; Methacrylic acid 2-hydroxyethyl ester; Ethylene glycol methacrylate;
Hydroxyethyl methacrylate; |
|

|
||
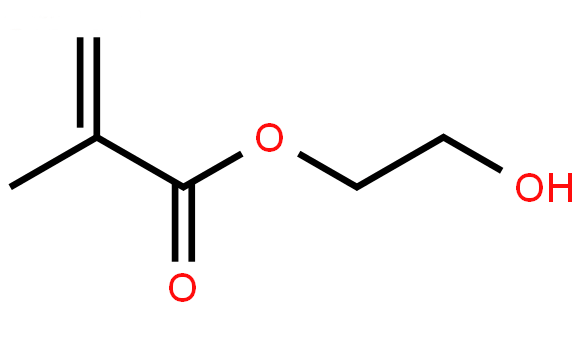
| Molecular Structure |
|
|
| Molecular Formula | C6H10O3 | |
| Molecular Weight | 130.14 | |
| CAS Registry Number | 868-77-9 | |
|
EINECS |
212-782-2 | |
| Properties | ||
| Density | 1.073 | |
| Melting point | -12 ºC | |
| Boiling point | 205-208 ºC | |
| Refractive index | 1.451-1.453 | |
| Flash point | 101 ºC | |
| Water solubility | soluble | |
| Safety Data | ||
| Hazard Symbols | Xi | |
| Risk Codes | R36/38;R43 | |
| Safety Description | S26;S28A | |
| Customs code | 29161490 | |
Hydroxyethyl methacrylate is mainly used for the modification of resins and coatings. Copolymerization with other acrylic monomers can produce acrylic resins with active hydroxyl groups in the side chains, which can undergo esterification and crosslinking reactions, synthesize insoluble resins and improve adhesion, and can be used as fiber treatment agents. It reacts with melamine-formaldehyde (or urea-formaldehyde) resin, epoxy resin, etc. to make two-component coatings. Adding to the high-end car paint can maintain the mirror gloss for a long time. It can also be used as an adhesive for synthetic textiles and medical polymer monomers.
Used in the production of resins for coatings, automotive topcoats and primers. It can also be applied to photopolymer resins, printing plates, inks, gels (contact lenses) and tinting material coatings, transmission electron microscopes (TEM) and optical microscopes ( LM) Embedding reagent, specially used for hydration samples of "sensitive antigen sites". GMA single system is white watery, viscous, thinner than water, and easier to penetrate than any resin and monomer. It is especially used to work on bones, cartilage and hard-to-penetrate plant tissues.
The plastics industry is used to manufacture acrylic resins containing active hydroxyl groups. Coating industry and epoxy resin, diisocyanate, melamine formaldehyde resin and other configurations are used to prepare two-component coatings. The oil industry is used as an additive for lubricating oil washing. In the electronics industry, it is used as a dehydration tool for electron microscopes. The textile industry is used to make adhesives for fabrics. Used as a chemical reagent in analytical chemistry. In addition, it is also used as a water-miscible embedding agent for the synthesis of medical polymer materials, thermosetting coatings and adhesives.
Mainly used in the manufacture of thermosetting coatings, fiber treatment agents, binders, photosensitive resins and medical polymer materials.
Hydroxyethyl methacrylate is a functional monomer for the preparation of thermosetting acrylic coating styrene butadiene rubber emulsion modifier. Acrylic modified polyurethane coatings, water-soluble electroplating coating binders, fiber finishing agents, paper coatings, photosensitive coatings and polyvinyl chloride resin modifiers are used in various resins for a wide range of applications.
1. It is derived from the reaction of ethylene oxide and methacrylic acid. Add ferric thiocyanate, tetramethyl ammonium chloride, etc. and a little hydroquinone to methacrylic acid, stir and heat to 90°C, under the protection of nitrogen, pass ethylene oxide gas to react for about 2h. Cool the reaction product to 60°C, add a small amount of hydroquinone and distill under reduced pressure, and collect 86-89°C (0.67kPa) fractions to obtain the finished product, with a yield of over 80%.
2. Potassium methacrylate and chloroethanol react in the presence of a polymerization inhibitor to produce crude 2-hydroxyethyl methacrylate, which is then salted out and refined to obtain a finished product.